Debunking Greenhouse Theory Physics
I have now published my updated theory of the atmosphere. Augmenting 19th Century Thermoelectric Greenhouse Theory with 20th Century Quantum Mechanics Raman Spectroscopy: Towards a Coherent Radiation Theory of the Atmosphere
Debunking Greenhouse Theory Physics
The Gassy Messenger.
Abstract
Modern climate science's fundamental premise (or assumption) is that greenhouse gases (around 2% of the atmosphere) absorb radiant infrared (IR) heat (as derived by IR spectroscopy) and are the main climate driver because of this speciality. This premise originates with the John Tyndall 1859 thermopile infrared detection experiment. The (other) non-greenhouse gases (N2 nitrogen and O2 oxygen) are distinguished from the greenhouse gases by their (said*) inability to absorb (infrared) heat, as deduced from the same experiment: here, absorption is confused with opacity. Raman spectroscopy (a complement to IR spectroscopy) challenges this greenhouse gas non-greenhouse gas paradigm and reveals this assumption and conclusion from any IR spectroscopy measurement to be false. It can be shown that N2 and O2 are, due to their symmetric vibration, totally transparent to all IR detectors but not transparent to Raman detectors. Ramon Spectroscopy shows CO2 and the other greenhouse gases to be typical and not special and that N2 and O2 are greenhouse gases. Further claims are also challenged with respect to CO2's unique properties in this entry. The only valid co-efficient or method to measure a gas's heat absorption is by Specific Heat Capacity, where CO2 is a poor contender.
Background
In an earlier entry, I claimed that CO2's heat-trapping property should but doesn’t repeat. Having found that CO2 doesn’t repeat (at least at any significant level to be measurable or notable), in this entry, I am attempting to explain why CO2's heat-trapping doesn’t repeat: why is it that we think it does. My conclusion is very disturbing: the foundation argument or premise of 'heat-trapping, climate-changing, and CO2 does not appear to be consistent with the related fundamental laws and textbook knowledge of physics. I have found all of the foundation arguments can be (easily) challenged, just by studying these laws in detail. Inspired by the work of Galileo, I am tempted to call this entry ‘The Gassy Messenger’ but have opted for the said Dark Climate.
Introduction
Below is a typical reference to a greenhouse effect definition:
Although Earth's atmosphere is 90% opaque to longwave IR radiation, the vast majority of it is not composed of gases that cause the greenhouse effect. Molecular nitrogen (N2) and oxygen (O2) make up roughly 98% of our atmosphere, and neither is a greenhouse gas. So, although the greenhouse effect is very powerful, a very small fraction of Earth's atmospheric gases generate it.
This greenhouse effect definition is developed and argued from the following experiments or theoretical claims (and others).
One by one, I shall attempt to address all of them in this entry.
1. The 1859 Tyndall experiment, which uncovered and determined specific atmospheric gases as IR 'absorbent', now known as the greenhouse gases;
1. The 1859 Tyndall experiment, which uncovered and determined specific atmospheric gases as IR 'absorbent', now known as the greenhouse gases;
3. CO2 heat chamber experiments: which demonstrate how the gas of CO2 temperature rises faster than 'air', when in isolation and when heated;
4. CO2‘s molecule structure: explanations suggesting it is the molecule structure (internal degrees of freedom) that determines the heat-trapping ability of CO2.
5. The far infrared re-emission (of heat energy).
6. Emphasis on Radiation implied low emphasis on conduction and convection.
A climate axiom is formed from these experiments and demonstrations, such as the greenhouse effect.
This axiom begs the question: if oxygen and nitrogen are non-greenhouse gases because they have no IR heat ‘blocking’/ absorbing signature, then how is the atmosphere warm at all?
The sea breeze used to be - and still is in any standard geography or aviation meteorology textbook - that when a ‘parcel’ of ‘air’ (which contains all the gases in the atmosphere) is heated by the land, it becomes less dense, rises, and this increasing draws cold air in from the sea. How can this sea breeze be explained when around 98% of the gases of air are non-heat absorbent and have no heat relationship?
From other similar paradoxes in physics: the mysterious Dark Energy and Dark Matter, I chose to term this climate greenhouse paradox ‘the dark climate', and its 'dark gases’, and have set out to try and explain how this paradox is so. Where have the gases of our atmosphere gone?’ Why are they thermal neutral? Either the greenhouse gas axiom is correct (and if this is so, we must accept this dark climate paradox), or the axiom is wrong. Their founding experiments are misinterpreted or misattributed.
In this (following) entry, I shall go through each experiment, one by one, and show that the assumption is wrong and that each experiment is either wrong, misinterpreted or misattributed. I shall conclude that the (total) atmosphere is made up of only Greenhouse gases – i.e. oxygen and nitrogen are also heat absorbent. I will show that CO2 is thermally typical and not at all special, and I will restore the textbook sea breeze explanation (not that it had changed).
1. The 1859 Tyndall experiment: IR spectroscopy
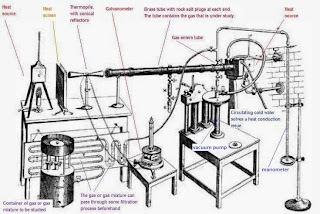
The – little known –1859 Tyndall experiment first identified and isolated what he interpreted to be what we now know as greenhouse gases. Below is a summary of his experiment; note that oxygen and nitrogen were found not to (what he thought at the time to) ‘absorb’ infrared. To this day, it is inferred by this experiment that greenhouse gases are, by nature, infrared absorbing.
Tyndall explained the heat in the Earth's atmosphere in terms of the various gases' capacities to absorb radiant heat, also known as infrared radiation. His measuring device, which used thermopile technology, is an early landmark in the history of absorption spectroscopy of gases.[7] He was the first to correctly measure the relative infrared absorptive powers of the gases nitrogen, oxygen, water vapour, carbon dioxide, ozone, methane, etc. (1859). He concluded that water vapour is the strongest absorber of radiant heat in the atmosphere and is the principal gas controlling air temperature. Absorption by the other gases is not negligible but relatively small. Before Tyndall, it was widely surmised that the Earth's atmosphere had a greenhouse effect, but he was the first to prove it. The proof was that water vapour strongly absorbed infrared radiation.[8] Relatedly, Tyndall, in 1860, was the first to demonstrate and quantify that visually transparent gases are infrared emitters.[9]
In the following clip Dr. Ian Stewart demonstrates the basic Tyndall experiment.
Tyndall’s experiment can easily be repeated, and his findings are reasoned in a modern context – just as we can Galileo’s 1609-1610 telescopic observations of the Moon, Jupiter, and Venus. The apparatus used is readily and relatively cheaply available in the form of the non-contact infrared thermometer or, by its more advanced relative, as shown in the clip above, the thermal imaging camera. Though these modern-day ‘gadgets’ are more advanced and adjustable than that available in Tyndall’s time, they operate using the same sensor technology, the thermopile.
Youtube clip of the thermopile:
The following gives some details about the thermopile and makes the link to today’s standard IR detectors.
A thermopile is an electronic device that converts thermal energy into electrical energy. It comprises several thermocouples connected in series or, less commonly, parallel.
Thermopiles do not respond to absolute temperature but generate an output voltage proportional to a local temperature difference or temperature gradient.
Thermopiles are used to provide an output in response to temperature as part of a temperature-measuring device, such as the infrared thermometers widely used by medical professionals to measure body temperature. They are also used widely in heat flux sensors (such as the Moll thermopile and Eppley pyrheliometer)[1][2][3] and gas burner safety controls. The output of a thermopile is usually in the range of tens or hundreds of millivolts.[4] In addition to increasing the signal level, the device may be used to provide spatial temperature averaging.[5]
.
Infrared Thermal Imaging Cameras or Infrared Cameras are essentially infrared radiation thermometers that measure the temperature at many points over a relatively large area to generate a two-dimensional image, called a thermogram, with each pixel representing a temperature.
1.1 Limitations of IR detectors
Today could be said to be the age of infrared. We use it in many applications, including meteorology and astronomy; it allows us to ‘see’ where we would otherwise be blind.
But to use it, the operator should understand the underlying (laws of) physics the IR instrument responds to. They must also understand its limitations, just as a pilot understands the limitations of an altimeter or compass and how they, too, can give misleading information.
To address this new knowledge and these limitations, all IR measuring instruments come with an operating manual that is readily available on the Internet. There are also training videos, such as the one below, on IR cameras and transparency.
To address this new knowledge and these limitations, all IR measuring instruments come with an operating manual that is readily available on the Internet. There are also training videos, such as the one below, on IR cameras and transparency.
These publications spell out (among other things such as opacity and transparency) that the instrument measures infrared radiation and not temperature and only reads what the instrument can ‘see’ (at the set frequency). This is to say: if at the set frequency of the instrument, something is opaque to IR, it can see it, and it can, therefore, measure it; and if something is transparent, it cannot see it and, therefore, cannot measure it.
Selective Emitters
“Infrared energy is an electromagnetic energy, just like visible light, radio waves, and x-rays. If I shine a flashlight at my chest it does not go through, but if I shine an x-ray at my chest it goes right through. The only difference between visible light and x-rays is the wavelength. So, by changing the wavelength of measurement, some objects may be more or less transparent at some wavelengths, and more or less opaque at others. Glass is a good example of this. Glass is transparent at short wavelengths, but is opaque at wavelengths longer than about 4.8 microns. Because glass is highly transparent at short wavelengths, this means that thin glass has a low emissivity value at short wavelengths. Because glass is highly opaque at long wavelengths, this means that glass has a high emissivity value at long wavelengths. The reflectivity of glass varies with wavelength, too. Glass is both opaque and highly non-reflective at wavelengths between about 6.6 and 8.2 microns, and so this is the wavelength band where glass has the highest emissivity value and where glass most closely approximates a blackbody material. (Page 7)
Thin film plastics are the classic example of selective emitters. These materials are made up of long chains of hydrogen and carbon atoms. The repetitive and uniform molecular structure of these materials means that the molecules and atoms all vibrate with a uniform series of harmonic frequencies. Infrared wavelengths coincident with these harmonic frequencies are preferentially absorbed (not reflected or transmitted) by the plastic material, and conversely, these materials emit infrared energy preferentially at the wavelengths coincident with these harmonic frequencies. When we look at a plastic sandwich bag we can see right through it, but if our eyes were filtered at 3.43 microns, which is the harmonic frequency for the H-C atomic bond, then the sandwich bag would appear completely opaque. When measuring the temperature of a selective emitter it is critical that a wavelength be selected to coincide with a strong emission band. This is a wavelength where the infrared emissions approach blackbody conditions, and where the material is highly opaque and non-reflective. Other examples of selective emitters are all gasses, and all highly transparent materials. Many crystalline materials, such as silicon and engineered ceramics, are also selective emitters. The uniformity and geometry of the molecular structure dictates the emissive nature of the material. Thin film coatings also act like selective emitters. In the metals industry, metal strips are often coated with a thin film. Oil-based paint, water-based paint, oil and wax are all examples of thin film coatings that can act like selective emitters. These materials are highly transparent at some wavelengths, and they are highly opaque and non-reflective at other wavelengths. The emissivity of the coated material is therefore highly influenced by the wavelength of measurement. The optimum wavelength of operation for an infrared thermometer will vary depending upon the coating material, the thickness, the required temperature range, and the need to view the coating or to view through the coating.
Without this theory, measurement would seem like a kind of magic, especially when measuring the temperature of a warm object through glass as opposed to through thin air. One only has to look at police night vision images of culprits hiding under plastic covers, thinking they are safe and hid—not so in the infrared.
In light of this theory and the application of modern-day instruments, the early Tyndall conclusions seem outdated: his conclusions need updating.
1.2 The Tyndall / Dr Stewart IR thermopile experiment revisited:
This clip, and the original 1859 Tyndall Experiment, is not a demonstration of heat absorption but rather a demonstration of the physical transition properties of (infrared) light and its effect on different substances.
We see the image of a flicking candle in the IR camera, and as the CO2 is let into the (sealed) cylinder, the bright candle image turns to a blue colour. It is concluded, just as Tyndall did, that the CO2 absorbs the infrared or is accentually trapping the heat from the candle. From the above literature and application of the instrument, an alternative conclusion should/could read: The bright candle image turns to a blue colour as the CO2 is opaque to the infrared at the frequency the camera is measuring, and the gases before the CO2 is let in are transparent at that frequency. To test this reasoning, we could have equally placed the glass in front of the candle and got the same or similar result as the CO2. It should be noted that Tyndall used rock salt crystals to contain the gases, which are transparent at that frequency. Rock salt is not used in the Stewart demonstration, but (IR transparent) thin plastic ‘clean full’ is. This can clearly be seen at a time. The image colour turned blue, showing the detector measuring the IR radiation emitted from the CO2 and, thus, its temperature. We could deduce from the colour of the CO2 that it is cold (which it should be coming from a pressurized state) or at least the temperature of the ambient gases it displaced.
If this interpretation is wrong, then we could equally conclude that window glass is an equally ‘greenhouse’ solid just as CO2 is a greenhouse gas. We don’t, it isn't.
2. N2 and O2 have no dipole, so they are not greenhouse gases.
This is the claim that excludes N2 and O2 as being a so-called greenhouse gas:
Nitrogen (N2) is symmetrical AND made of identical atoms.Even with rotation or vibration, there is no unequal sharing of electrons between one N atom and the other. So N2 has no dipole, and an EM photon passes by without being absorbed. Similarly, for O2. reference
Yes, N2 and O2 are both transparent to IR spectroscopy, but this fact begs the question (as stated above): How can the atmosphere be warm if 98% of it (N2 and O2) are not IR (heat) ‘absorbent'? How can N2 and O2 be non-GHG gases, yet they have a heat capacity coefficient?
Something must be wrong with this conjecture.
To solve this paradox, an alternative measuring instrument or method other than IR spectroscopy must be sourced to reveal the actual IR properties of N2 and O2 (and all other gases). Such an instrument does exist, Raman spectroscopy.
2.1 Raman Spectroscopy
Raman Spectroscopy is a known complement to IR spectroscopy for analysing the vibrational properties of substances: it ‘sees’ what IR spectroscopy can't. reference
The following clips explain Raman spectroscopy well. I suggest you play them more than once to yourself, as they are very insightful and offer a perfect solution to the dark climate paradox.
Youtube clip on Raman spectroscopy
The following clips explain Raman spectroscopy well. I suggest you play them more than once to yourself, as they are very insightful and offer a perfect solution to the dark climate paradox.
2.2 N2 and O2 Raman Spectroscopy
A hypothesis was set: N2 and O2 have an infrared signature. To confirm this hypothesis, an experiment with a sample of the atmosphere would be conducted to measure N2 and O2 in the IR region of the EM spectrum, or secondary research would point to a similar result. In the absence of an experiment, secondary results were searched using a Google image search with the keywords Raman spectroscopy atmosphere. A positive image ('Fig. 11' below) was quickly found. This figure and its caption clearly come from an unrelated journal publication. Still, the image reveals what many others in the same search reveal - such as Heat Treating: Proceedings of the 16th Conference.Jon L. Dossett, Robert E. Luetje, 1996 page 228.
'Figure 11: Resonance Raman spectrum from outdoor measurement on nitro methane in the vapour phase at an irradiation wavelength of 220 nm. The sample temperature was approximately 328 K, the outdoor temperature was 274 K, and the atmospheric pressure was about 755 Torr. The spectrum was accumulated during 1000 laser pulses.'http://www.hindawi.com/journals/ijs/2012/158715/fig11/
Notable are the O2 and N2 peaks at wavenumbers 1556cm-1 and 2331cm-1, respectively. These wavenumbers correspond to wavelengths 6.43 microns and 4.29 microns, respectively, in the near-infrared region of the EM.
Another image found is below (Fig. 18), showing again the 1556 O2 and other peaks at higher wavelengths along the spectrum.
Fig. 8. Another image found is below (Fig. 18), showing again the 1556 O2 and other peaks at higher wavelengths along the spectrum.
UV Raman spectra are shown at 300 and 93 K in 18O2 atmosphere for the Fe/MFI sample exchanged with NaOH and then subsequently exchanged with NH4+ and reduced in hydrogen. At 300 K the band corresponding to peroxide oxygen increases and the band corresponding to superoxide decrease relative to their intensities at 93 K (51). http://www.sciencedirect.com/science/article/pii/S0360056406510028
From these images, it can be concluded that N2 and O2 (and other gases) are infrared opaque or absorbent and are too greenhouse gases.
To verify that the above observation is rational, and predictable a cause or explanation to these 'peaks' should be sourced. To do this, I had to show that the vibration modes for both N2 and O2 were symmetrical. As the quote at the top of this section said, 'Nitrogen (N2) is symmetrical AND made of identical atoms' I had part of an answer, but with another Google search, I found this directly to my question academic reference: Chemistry 362 Dr. Jean M. Standard.
3 . Are the stretching modes of the diatomic molecules O2 and N2 infrared active? Why or
why not? Are the stretching modes of O2 and N2 Raman active? Why or why not?
The stretching mode of a homonuclear diatomic molecule does not lead to a change in the dipole moment of the
molecule; hence, the stretching mode is not IR active.
The stretching mode of a homonuclear diatomic molecule does lead to a change in polarizability of the molecule;
hence, the stretching mode is Raman active. Another way to consider this is that since O2 and N2 possess
centers of symmetry, the stretching mode must be Raman active because it is IR inactive.
It should be noted that the very fact that Nitrogen (N2) is symmetrical AND made of identical atoms is the reason it is transparent to IR spectroscopy: it is symmetrical by nature and so will never show up as a law of physics, even in part, like other molecules such as CO2.
2.3 Conclusion
N2 and O2 are not at all IR transparent; they are instrumentation and knowledge problems.
If this Raman spectroscopy discovery is true, then the 2% (volume) of said greenhouse gases should be revised and relegated to 100% by adding N2 and O2 (and others if so). Any assumptions or premises made by any climate models, climate knowledge, or claims that the atmosphere consists of around 2 % (volume) special greenhouse gases will need to be reviewed—as said above.





I think you make some errors in this post. There are two fundamental premises about CO2. Firstly, that it traps heat in the atmosphere. Heat by definition cannot be trapped. But more importantly, the trapped heat turns out not to be trapped because it is said to return to the surface and cause additional heating. The two premises are essential for the theory of human caused global warming. It is of course impossible firstly, for heat to be trapped anywhere (except perhaps as latent heat but this is not relevant with CO2) and secondly, for heat to be transferred from the colder atmosphere to the warmer surface.
ReplyDeleteI think you made a mistake with the quote of the greenhouse effect. A better quote is just before this paragraph in the reference and it is: "The greenhouse gases act as a blanket covering Earth's surface; a lot of energy flows back and forth between the insulating blanket and the "body" of the planet beneath; but relatively little escapes from this efficient insulating cover." It is just utter nonsense. Heat does not flow back and forth. Heat always travels from a high temperature to a lower temperature. In any case insulation does not increase the temperature of anything. It cannot because it does not spontaneously generate thermal energy or do work. All insulation does is to reduce the energy needed to maintain a specific temperature. The article you refer goes on to discuss what I refer to as back-radiation. This is the idea that radiation leaves the surface, is trapped by CO2 and then returns to the surface causing additional heating. This is not possible. Although radiation will be exchanged, radiation is not the same as thermal energy transfer and there is no mechanism by which an object can heat itself by returning radiation. The quote exposes a fundamental issue. If, as they say, very little heat escapes, then how can they claim that there is an energy balance at the surface of the atmosphere.
I believe you miss an essential point in this article and that is the so called energy balance diagram is no such thing. Look at the quantities. They are not energy but energy flux (w/m2). Flux is not a conserved quantity. The area associated with the flux from the sun is half the earth's surface. The surface associated with the energy leaving is the entire surface. By equating the fluxes the energy leaving is doubled and hence the need for the nonsense theory of back radiation. They also assume a balance at the surface for which there is no justification and this produces more heat from nowhere.
You also miss another point when discussing the 2% of greenhouse gases. It is only the human increases of CO2 that are said to cause the warming. This has been about 100ppm. Effectively 1 extra molecule of CO2 out of every 10,000 is causing the warming of the surface. Your rational discussion is pointless when the entire issue of AGW is completely wrong and irrational.
AGW is not about science, it is about money. People benefit from taxation to develop renewable energy and scientists who will only get government funding if they waste their time on pointless research based on nonsense. I note that you have challenged Brian Cox on an issue. I suggest you look for the YouTube video of his appearance on the Australian TV programme "Q&A" in 2016 where he discussed climate change with Malcolm Roberts. Cox's evidence was that there is a consensus of scientists who are in agreement. I wonder if he would claim a consensus of scientists agree that the Higgs Boson particle exists. Of course not, it was based on experimental evidence. He also held up graphs of CO2 and temperature claiming that the apparent correlation was evidence of causation. He has been bought by money.
Thank you for the feedback. I have now published my updated theory of the atmosphere.
DeleteQuantum Mechanics and Raman Spectroscopy Refute Greenhouse Theory
https://www.researchgate.net/publication/328927828_Quantum_Mechanics_and_Raman_Spectroscopy_Refute_Greenhouse_Theory
and
The Greenhouse Gases and Infrared Radiation Misconceived by Thermoelectric Transducers
https://www.researchgate.net/publication/329311153_The_Greenhouse_Gases_and_Infrared_Radiation_Misconceived_by_Thermoelectric_Transducers
Blair
I’m now not positive where you’re getting your information, but good topic. I needs to spend some time finding out much more or figuring out more. Thank you for great info I used to be searching for this info for my mission. greenhouse
ReplyDelete